In a significant leap toward malaria eradication, India is developing a multi stage malaria vaccine candidate called AdFalciVax. This landmark project is a collaboration between the Indian Council of Medical Research (ICMR) and the Department of Biotechnology – National Institute of Immunology (DBT-NII). With India accounting for a substantial portion of global malaria cases, this initiative marks a crucial stride in bolstering the country’s indigenous healthcare capabilities.
What is AdFalciVax?
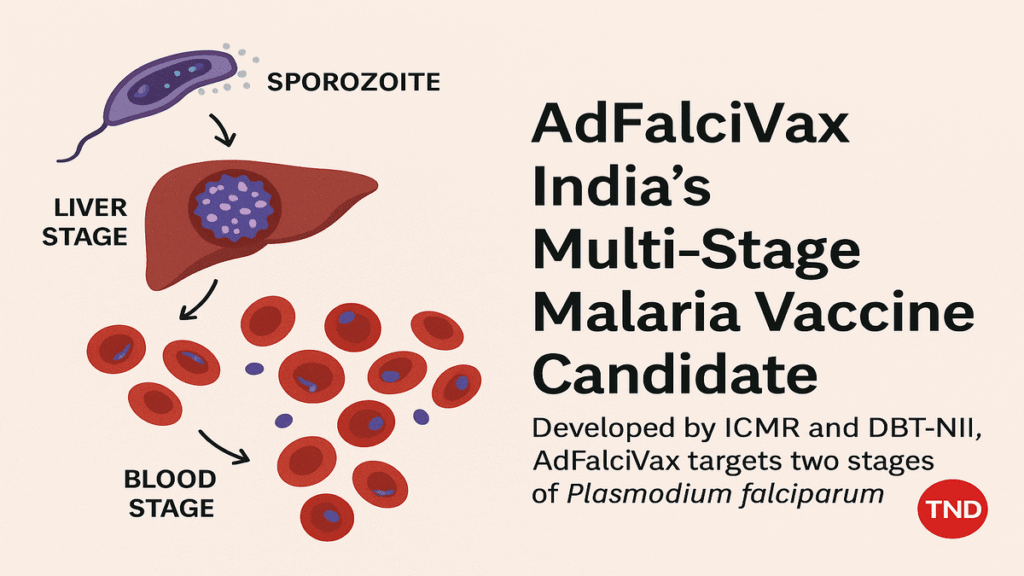
AdFalciVax is a recombinant chimeric malaria vaccine candidate engineered to combat the deadliest form of malaria caused by the Plasmodium falciparum parasite. What sets AdFalciVax apart is its multi stage mechanism, designed to target two critical stages in the parasite’s life cycle the liver stage (pre-erythrocytic) and the blood stage (erythrocytic). These stages are vital for parasite multiplication and transmission.
Unlike single stage malaria vaccines currently in global use, AdFalciVax aims to both protect individuals from infection and reduce community level parasite transmission, making it a dual action vaccine. This could be a game changer in the broader effort to eliminate malaria in endemic regions.
Indigenous Innovation in Malaria Vaccine Development
This is India’s first indigenous multi stage malaria vaccine, demonstrating the country’s growing scientific capabilities. The development of AdFalciVax reflects the “Make in India” initiative’s impact on high stakes biomedical research. It also aligns with India’s commitments under the Global Technical Strategy for Malaria 2016–2030, which targets a 90% reduction in malaria incidence and mortality.
Both ICMR and DBT-NII are playing a leading role, with the National Institute of Immunology leveraging years of structural biology and molecular immunology research to design the recombinant vaccine.
Scientific Approach Behind AdFalciVax
AdFalciVax is based on a recombinant adenoviral vector platform, which is genetically engineered to express antigens from the Plasmodium falciparum parasite. These antigens stimulate the human immune system to develop a strong and durable response. The dual target approach increases the chances of stopping the parasite at different stages before it can mature in the liver or multiply in the bloodstream.
This multi stage malaria vaccine concept also offers the added advantage of reducing mosquito to human transmission, providing community wide protection and breaking the chain of infection in endemic zones.
Why India Needs a Malaria Vaccine Now
According to the World Malaria Report 2023, India reported over 1.2 million confirmed malaria cases, though the actual burden could be higher due to underreporting. With climate change expanding mosquito habitats, malaria continues to pose a serious public health risk in several Indian states, particularly in tribal and rural regions.
Traditional methods like insecticide treated nets and indoor spraying have helped, but they are not foolproof. A multi stage malaria vaccine like AdFalciVax could offer a sustainable and long term solution by supplementing existing preventive tools.

Global Context: India’s Entry into the Malaria Vaccine Race
Globally, malaria vaccine efforts have been led by RTS,S/AS01 (Mosquirix) and R21/Matrix-M, with mixed efficacy results and logistical challenges. Both primarily target the pre-erythrocytic stage. However, AdFalciVax’s dual stage targeting gives it a strategic advantage, especially in high transmission regions.
India’s move to develop its own malaria vaccine not only strengthens its health security but also positions it as a potential vaccine exporter to sub-Saharan Africa and Southeast Asia, where Plasmodium falciparum continues to claim lives.
Current Development Phase and Road Ahead
AdFalciVax is currently in preclinical evaluation stages, with animal model trials being conducted to assess safety, immunogenicity, and efficacy. Once completed, clinical trials in humans are expected to begin under ICMR’s supervision, following approval from the Drug Controller General of India (DCGI).
If successful, India could witness the launch of its first home grown malaria vaccine by 2027, depending on regulatory clearances and manufacturing scale up.
Expert Opinions
Dr. Rajni Kant, head of the ICMR’s Malaria Research Program, stated:
AdFalciVax embodies India’s ambition to lead global public health innovation. A multi stage malaria vaccine will not only protect individuals but help eliminate malaria as a public health threat in India.”
Similarly, DBT-NII scientists have emphasized that this multi-stage vaccine approach could reduce dependency on imported vaccines, ensuring affordability and accessibility for millions of Indians.
Challenges Ahead
Despite the scientific promise, scaling up vaccine production, ensuring cold chain logistics in remote areas, and addressing vaccine hesitancy remain key hurdles. Equally important is the need for ongoing government support and public private partnerships to accelerate development and deployment.
Conclusion
The development of AdFalciVax, India’s first multi stage malaria vaccine, represents a pivotal step in the nation’s fight against one of its oldest diseases. With high hopes pinned on its success, the collaboration between ICMR and DBT-NII is not just about scientific advancement, but about saving lives and achieving equitable health outcomes.
As India moves closer to malaria elimination, AdFalciVax could be the game changing solution the country and the world have been waiting for.
Stay Connected with The News Drill for more updates.
Source: ICMR







